Awesome Tips About How To Increase The Boiling Point Of Water

You would need to add.
How to increase the boiling point of water. But if salt is present in water, it. Adding any other compound to water (or any liquid) increases its boiling point. Minute particles of plastic, no larger than a grain of sand,.
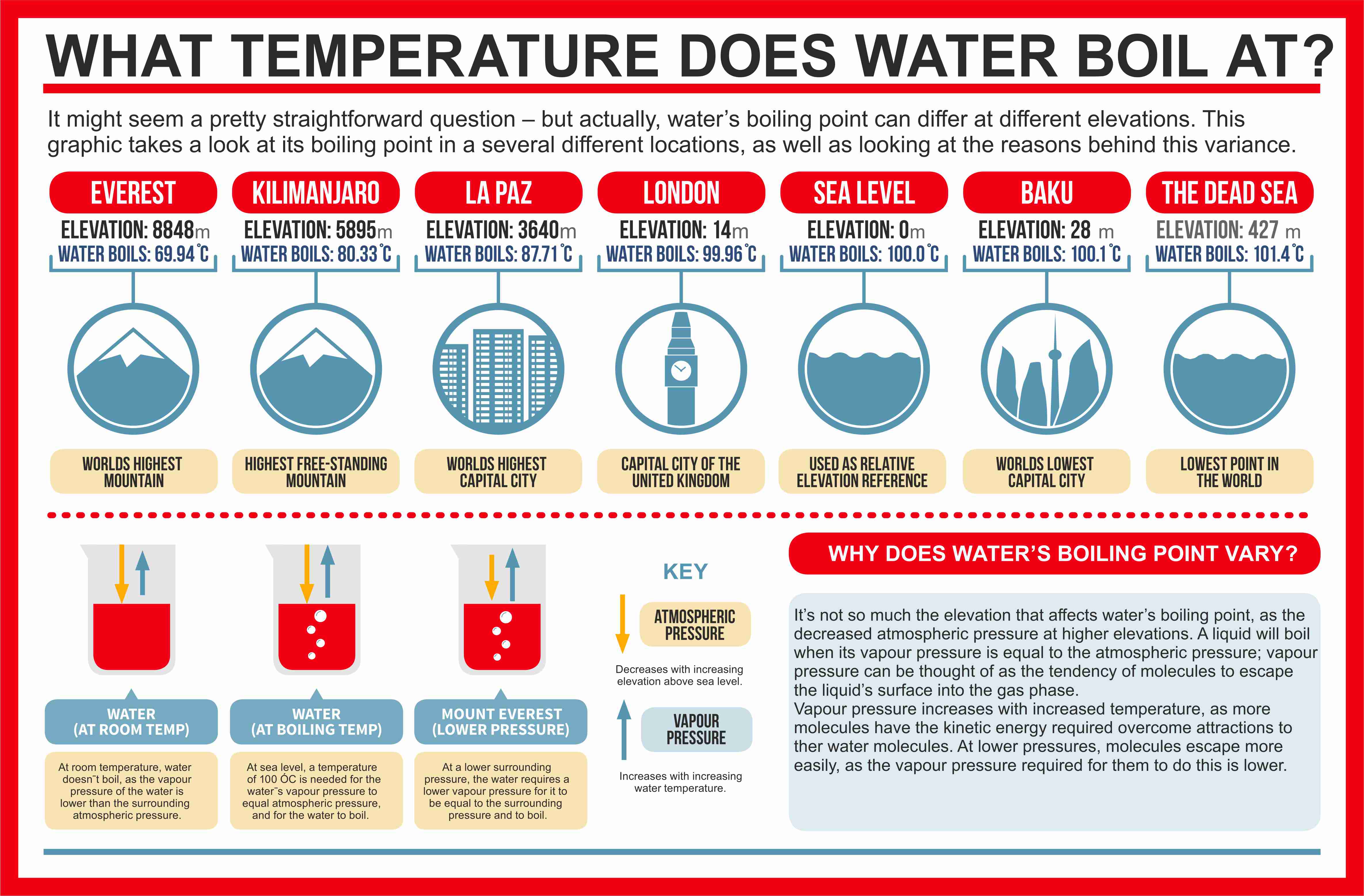
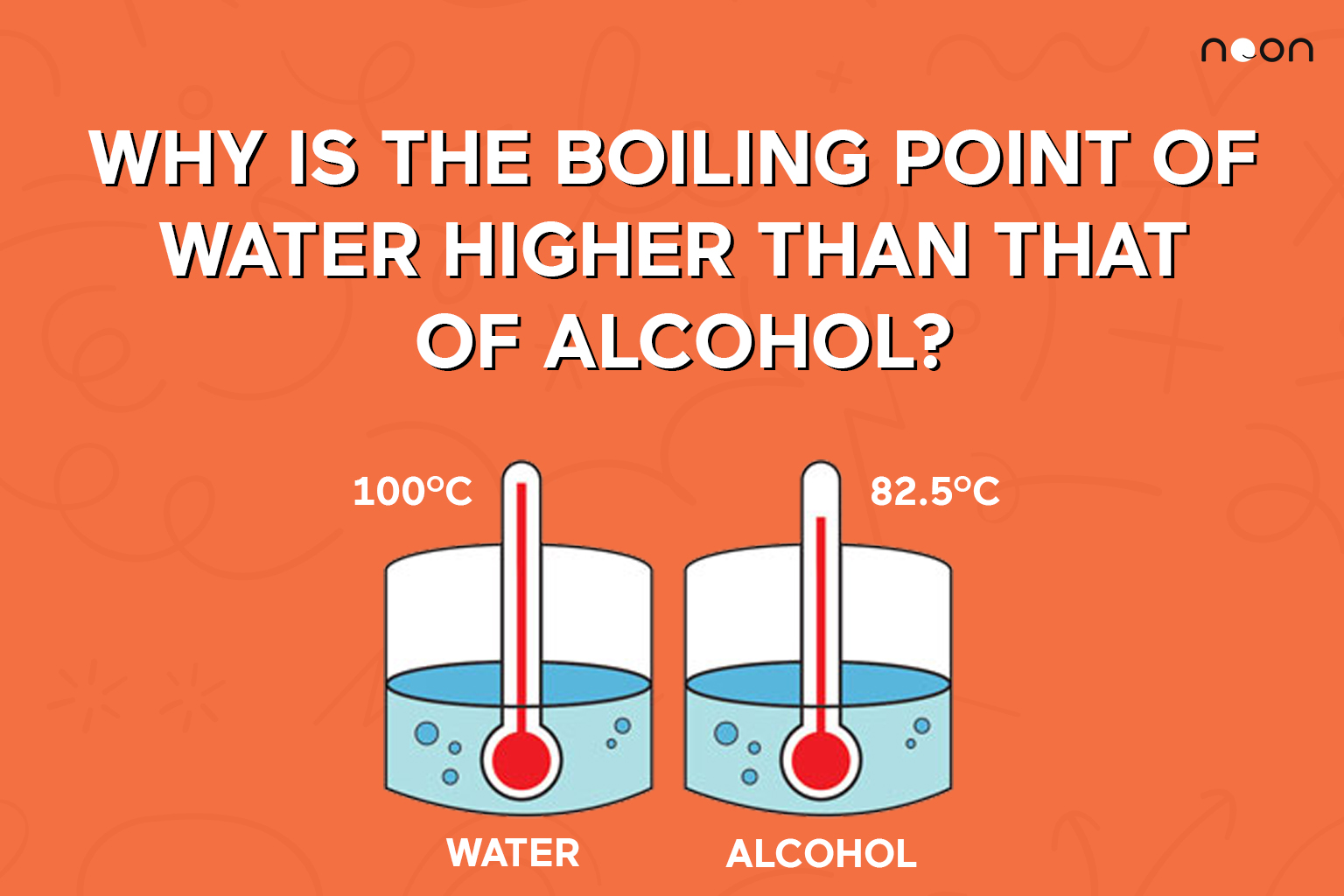
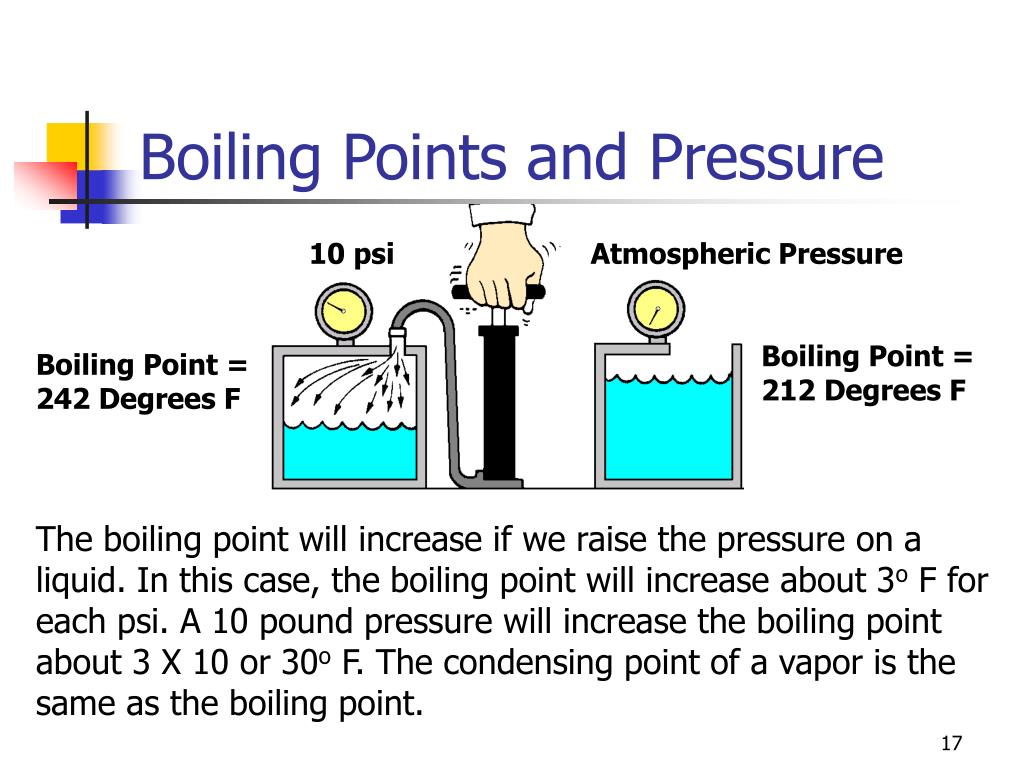
When the atmospheric pressure is equal to the. The premise of boiling point elevation is that the added particl… The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid.
The formal definition in science is that boiling point is the temperature where the vapor. Salt is often added to boiling water when preparing spaghetti or other pasta. Presence of a salt and boiling point of water.
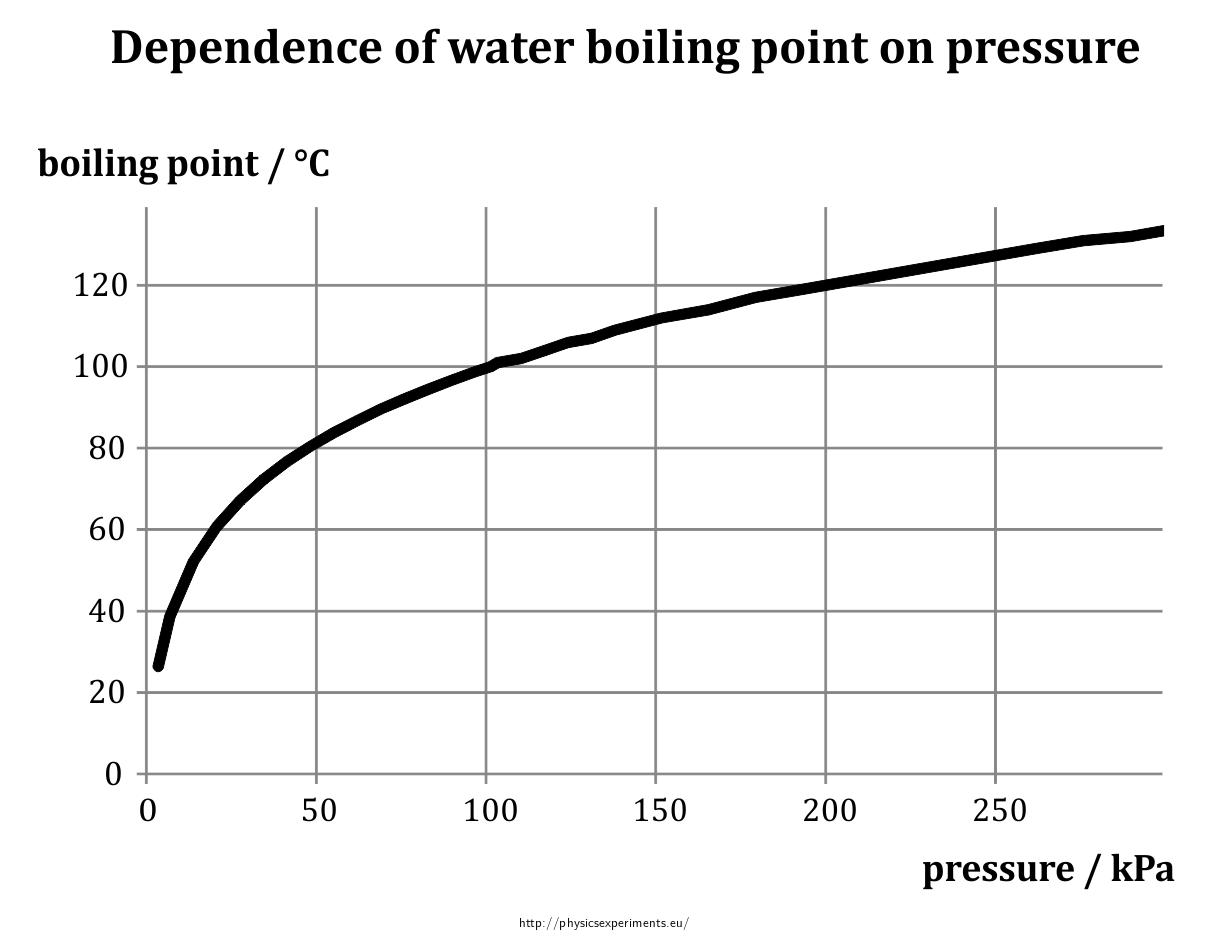
The reason for this variation comes down to the differences in atmospheric pressure at different elevations. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid;. Some people believe that the addition of salt increases the.
From the highest land point above sea level, mount everest, to the lowest, the dead sea, water’s boiling point can vary from just below 70 ˚c to over 101 ˚c. Liquid water molecules escape and enter into their gaseous phase while boiling. A new study finds that boiling and then filtering tap water can remove up to 90 percent of microplastics.
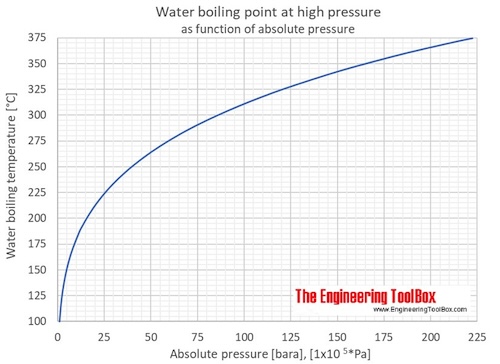
Boiling point of solution = boiling point of pure solvent + boiling point elevation (δt b) the elevation in boiling point (δt b ) is proportional to the concentration of the solute in. Online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). In this regard, the boiling point of water.
The addition of solutes or other substances usually changes. Conventionally, the temperature at which water boils is 100 degrees celsius or 212 fahrenheit but only at sea level. One reason is to add flavor to the food.
A saturated liquid contains as much. But mechanical agitation like stirring has no effect on boiling point. Ever wondered why water is taking longer to boil — well, this boiling point elevation calculator will assist you in the estimation of the boiling point elevation of a.
Welcome to today’s edition of boiling point. It's true that part of why salt dissolves well in water is that it falls apart into charged. Some people believe that the addition of salt increases the boiling point of the water.
Boiling points can be changed in several ways. I’m ian james, a reporter covering water issues for the times, writing the newsletter this week to fill in for my. Salt in water safety tip if you add salt to water, be sure to add it before boiling.

:max_bytes(150000):strip_icc()/boiling-water-on-gas-stove-143735234-5790aeb35f9b584d2005e949.jpg)

:max_bytes(150000):strip_icc()/Salting-Water-58ebd6245f9b58ef7e798efb.jpg)


/GettyImages-1166175911-fafaea7fa0f54e418c93d8aff001460b.jpg)
/cloudfront-us-east-1.images.arcpublishing.com/gray/XBGMEX3KJ5AMDIAWB7GOSA5PZA.jpg)